Use the data given for the substances listed below to answer the question that follows on its physical state at its room temperature of and atmospheric pressure.
Substance
Melting point/
Boiling point/
sodium
98
883
radon
-71
-62
ethanol
-117
78
cobalt
1492
2900
nitrogen
-210
-196
propane
-188
-42
ethanoic acid
16
118
Which substance is a liquid over the smallest range of temperature?

Important Questions on The Nature of Matter
Use the data given for the substances listed below to answer the question that follows on its physical state at its room temperature of and atmospheric pressure.
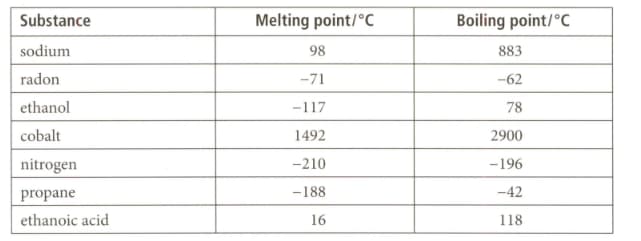
Which two substances are gaseous at .
Use the data given for the substances listed below to answer the question that follows on its physical state at its room temperature of and atmospheric pressure.
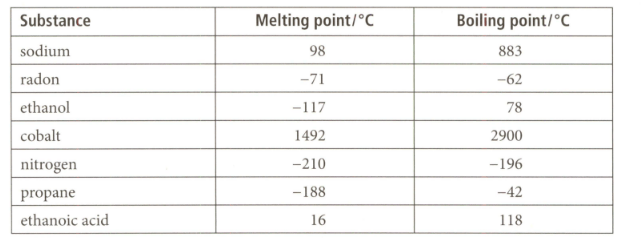
Which substance has the lowest freezing point?
and atmospheric pressure.
| Substance | Melting point/°C | Boiling point/°C |
| sodium | 98 | 883 |
| radon | –71 | –62 |
| ethanol | –117 | 78 |
| cobalt | 1492 | 2900 |
| nitrogen | –210 | –196 |
| propane | –188 | –42 |
| ethanoic acid | 16 | 118 |
Use the data given for the substances listed below to answer the question that follows on its physical state at its room temperature of and atmospheric pressure.
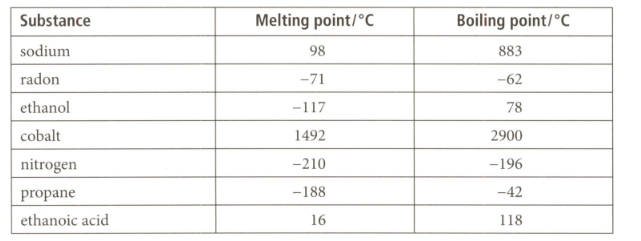
A sample of ethanoic acid was found to boil at at atmospheric pressure. Use the information in the table to comment on this result.
Choose from the words below to fill in the gaps in the passage. Words may be used once, more than once, or not at all.
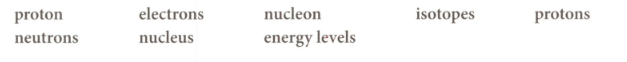
Atoms are made up of three different particles _____ which are positively charged; _____ which have no charge; and _____ which are negatively charged.
The negatively charged particles are arranged in different _____ (shells) around the _____ of the atom. The particles with a negligible mass are the _____. Atoms of the same element with different numbers of _____ are known as _____.
This part of the exercise is concerned with electron arrangements and the structure of the Periodic Table. Complete these sentences by filling in the blanks with words or numbers.
The first shell fills up first from _____ to helium.
This part of the exercise is concerned with electron arrangements and the structure of the Periodic Table. Complete these sentences by filling in the blanks with words or numbers.
The second shell fills next from lithium to _____.
This part of the exercise is concerned with electron arrangements and the structure of the Periodic Table. Complete these sentences by filling in the blanks with words or numbers.
Eight _____ go into the third shell from sodium to argon.
