Use the letters only written in the periodic table given below to answer the questions that follow
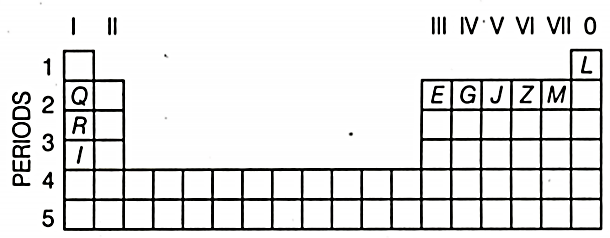
(i) State the number of valence electrons in atom .
(ii) Which element forms ions with a single negative charge?
(iii) Out of and which one is more reactive?
(iv) Which element has its electrons arranged in four shells?

(i) State the number of valence electrons in atom .

Important Questions on Periodic Properties and Their Variations
The metals of the group from top to bottom are and
(i) Which one of these elements will form ions most readily and why?
(ii) State the common feature in the electronic configuration of all these elements.
(Increasing order of metallic character).
Arrange the following as per the instructions given in the bracket.
(Decreasing order of atomic size).
Arrange the following as per the instructions given in the bracket.
(Increasing order of ionisation energy).
(Increasing order of electron affinity).
(i) A basic oxide.
(ii) An oxide which dissolves in water forming an acid.
(iii) An amphoteric oxide.
(iv) A covalent oxide of a metalloid.
Element is a metal with a valency is a non-metal with a valency .
(i) Write an equation to show how forms an ion?
(ii) If is a diatomic gas, write an equation for the direct combination of and to form a compound.
The amount of energy released when an atom in the gaseous state accepts an electron to form an anion.
