Use your copy of the periodic table to help you answer the question.
Fluorine and astatine are halogens. Use your knowledge of the other halogens to predict the similarities in chemical properties of fluorine and astatine.

Important Questions on Cambridge IGCSE Exam Questions from Paper 3
Across the world, food safety agencies are investigating the presence of minute traces of the toxic hydrocarbon, benzene, in soft drinks. It is formed by the reduction of sodium benzoate by vitamin C.
Sodium benzoate is a salt. It has the formula . It can be made by neutralising benzoic acid using sodium hydroxide. Deduce the formula of benzoic acid.
Across the world, food safety agencies are investigating the presence of minute traces of the toxic hydrocarbon, benzene, in soft drinks. It is formed by the reduction of sodium benzoate by vitamin C.
Sodium benzoate is a salt. It has the formula . It can be made by neutralising benzoic acid using sodium hydroxide. Write the word equation for the reaction between benzoic acid and sodium hydroxide.
Across the world, food safety agencies are investigating the presence of minute traces of the toxic hydrocarbon, benzene, in soft drinks. It is formed by the reduction of sodium benzoate by vitamin C.
Sodium benzoate is a salt. It has the formula . It can be made by neutralising benzoic acid using sodium hydroxide. Name two other compounds that would react with benzoic acid to form sodium benzoate.
Benzene conatins of carbon and its relative molecular mass is . What is the percentage of hydrogen in benzene?
Benzene contains of carbon and its relative molecular mass is . Calculate the ratio of moles of atoms: moles of atoms in benzene.
Benzene conatins of carbon and its relative molecular mass is . Calculate the empirical formula and molecular formula of benzene.
This shows the structural formula of Vitamin C.
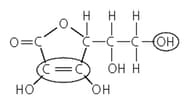
What is its molecular formula?
This shows the structural formula of Vitamin C.
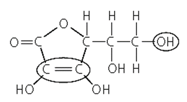
Name the two functional groups that are circled.
