MEDIUM
JEE Main
IMPORTANT
Earn 100
Using very little soap while washing clothes, does not serve the purpose of cleaning of clothes, because
(a)soap particles remain floating in water as ions.
(b)colloidal structure of soap in water is completely disturbed.
(c)the hydrophobic part of soap is not able to take away grease.
(d)the micelles are not formed due to concentration of soap, below its value.
50% studentsanswered this correctly

Important Questions on Surface Chemistry
EASY
JEE Main
IMPORTANT
Match List I with List II.
| List-I Enzyme |
List-II Conversion of |
||
| A. | Invertase | I. | Starch into maltose |
| B. | Zymase | II. | Maltose into glucose |
| C. | Diastase | III. | Glucose into ethanol |
| D. | Maltase | IV. | Cane sugar into glucose |
Choose the most appropriate answer from the options given below
MEDIUM
JEE Main
IMPORTANT
of is adsorbed on of platinum powder at and bar pressure. The volume of the gas adsorbed per gram of the adsorbent is
(Given : bar )
HARD
JEE Main
IMPORTANT
Match List - I with List - II.
| List-I | List-II | ||
| A | Lyophilic colloid | I | Liquid-liquid colloid |
| B | Emulsion | II | Protective colloid |
| C | Positively charged colloid | III | |
| D | Negatively charged colloid | IV | hot water |
Choose the correct answer from the options given below
MEDIUM
JEE Main
IMPORTANT
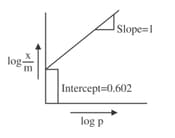
If the initial pressure of a gas is , the mass of the gas adsorbed per gram of the adsorbent is____
MEDIUM
JEE Main
IMPORTANT
The Zeta potential is related to which property of colloids?
EASY
JEE Main
IMPORTANT
Match List-I with List-II
| List-I | List-II | ||
| (A) | negatively charged sol | (I) | |
| (B) | macromolecular colloid | (II) | sol |
| (C) | positively charged sol | (III) | Starch |
| (D) | Cheese | (IV) | a gel |
Choose the correct answer from the options given below
MEDIUM
JEE Main
IMPORTANT
Which of the following is a correct statement?
HARD
JEE Main
IMPORTANT
(w/v) solution of causes precipitation of a certain sol in hours. The coagulating value of for the sol is
[Given : Molar mass : ]
