HARD
JEE Main/Advance
IMPORTANT
Earn 100
of solution (Resistance ) is mixed with of solution (Resistance ). Resistance of final solution is then will be (using same cell)
(a)
(b)
(c)
(d)
60% studentsanswered this correctly

Important Questions on Electrochemistry
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
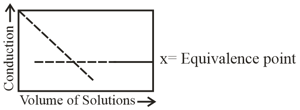
The following curve for the conductometric titration is obtained when -
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
