HARD
JEE Main
IMPORTANT
Earn 100
Van der Waal's equation for a gas is stated as,
.
This equation reduces to the perfect gas equation, When,
(a)temperature is sufficiently high and pressure is low.
(b)both temperature and pressure are very low.
(c)both temperature and pressure are very high.
(d)temperature is sufficiently low and pressure is high.
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
HARD
JEE Main
IMPORTANT
A graph of vapour pressure and temperature for three different liquids and is shown below:
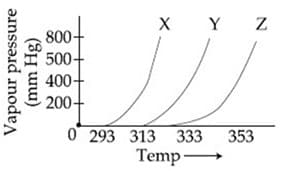
The following inferences are made:
has higher intermolecular interactions compared to
has lower intermolecular interactions compared to
has lower intermolecular interactions compared to
The correct inferences is/are:
EASY
JEE Main
IMPORTANT
The predominant intermolecular forces present in ethyl acetate, a liquid, are:
EASY
JEE Main
IMPORTANT
Among the following, the incorrect statement is:
MEDIUM
JEE Main
IMPORTANT
Identify the correct labels of and in the following graph from the options given below:
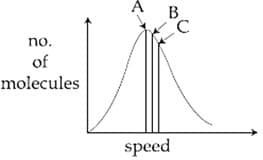
Root mean square speed most proable speed Average speed
MEDIUM
JEE Main
IMPORTANT
At the vapour pressure of is and that of acetone is A solution of in acetone has a total vapour pressure of . The false statement amongst the following is:
EASY
JEE Main
IMPORTANT
The relative strength of the interionic/ intermolecular forces in a decreasing order is:
EASY
JEE Main
IMPORTANT
For gaseous state, if most probable speed is denoted by , average speed by and root mean square speed by , then for many molecules, what is the ratios of these speeds?
HARD
JEE Main
IMPORTANT
If Z is the compressibility factor, then Van der Waal's equation at low pressure can be written as:
