Vapour density of the equilibrium mixture of and is found to be : For the equilibrium .
Calculate the abnormal molecular weight.
Important Questions on Chemical Equilibrium
Vapour density of the equilibrium mixture of and is found to be : for the equilibrium .
Calculate: degree of dissociation.
Vapour density of the equilibrium mixture of and is found to be , for the equilibrium .
Calculate the percentage of in the mixture.
Vapour density of the equilibrium mixture of and is found to be : for the equilibrium
Calculate for the reaction if total pressure is .
Two gases and are present in two containers at separated by a narrow tube of negligible volume having valve in between.
On opening the valve, the reaction attains equilibrium at . If , at the concentration of at equilibrium is:
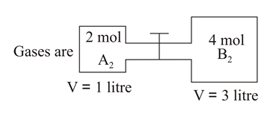
at constant temperature. The ratio between the initial moles and final moles is
