EASY
Earn 100
Vapour pressure of a saturated solution of a sparingly soluble salt is of at . If vapour pressure of pure water is of at , calculate the solubility product of at .
Important Questions on Solutions
MEDIUM
MEDIUM
MEDIUM
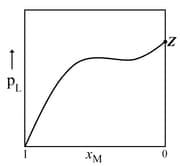
MEDIUM
HARD
EASY
MEDIUM
MEDIUM
HARD
HARD
(Given that the vapour pressure of pure liquid A is at temperature )
MEDIUM
EASY
MEDIUM
MEDIUM
(
)
HARD
HARD
Benzene and toluene form an ideal solution over the entire range of composition. The vapour pressure of pure benzene and toluene at are and , respectively. What is the mole fraction of toluene in vapour phase when of benzene is mixed with of toluene?
(Molar mass of benzene and toluene are and , respectively)
MEDIUM
MEDIUM
HARD
[Given ]
EASY

