Water at is poured into a test tube whose bottom is immersed in a large amount of water at . As a result, the water in the test tube is heated to during a time . Then water at is poured into the test tube whose bottom is immersed in a large amount of water at . The water in the test tube is cooled to during a time .
What time is longer: or ?

Important Questions on Heat and Molecular Physics
water in the vessel. The vessels are heated to the boiling point of water and left to cool. The time of cooling for the vessel with the ball to the temperature of the ambient is known to be times as long as the time of cooling for the vessel without a ball.
Determine the ratio of the specific heats of the ball material and water.
Two identical thermally insulated cylindrical calorimeters of height are filled to one-third. The first calorimeter is filled with ice formed as a result of freezing water poured into it, and the second is filled with water at . Water from the second calorimeter is poured into the first one, and as a result it becomes to be filled to two-thirds. After the temperature has been stabilised in the first calorimeter, its level of water increases by . The density of ice is , the latent heat of fusion of ice is , the specific heat of ice is , and the specific heat of water is .
Determine the initial temperature of ice in the first calorimeter.
Determine the mass of ice which melts in the process and the work done by an external force if it is known that the pressure required to decrease the fusion temperature of ice by is , while the pressure required to reduce the volume of a certain mass of water by is .
(1) Solve the problem, assuming that water and ice are incompressible.
(2) Estimate the correction for the compressibility, assuming that the compressibility of ice is equal to half that value for water.
For many substances, there exists a temperature and a pressure at which all the three phases of a substance (gaseous, liquid, and solid) are in equilibrium. These temperature and pressure are known as the triple point. For example, and for water. The latent heat of vaporization of water at the triple point is , and the latent heat of fusion of ice is .
Find the latent heat of sublimation (i.e. a direct transition from the solid to the gaseous state) of water at the triple point.
The saturated vapour pressure above an aqueous solution of sugar is known to be lower than that above pure water, where it is equal to by ,
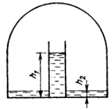
where is the molar concentration of the solution. A cylindrical vessel filled to height with a sugar solution of concentration is placed under a wide bell. The same solution of concentration is poured under the bell to a level (Fig).
Determine the level of the solution in the cylinder after the equilibrium has been set in. The temperature is maintained constant and equal to . The vapour above the surface of the solution contains only water molecules, and the molar mass of water vapour is .
Determine the fraction of the duct filled with molten metal.
The shell of a space station is a blackened sphere in which a temperature is maintained due to the operation of appliances of the station. The amount of heat given away from a unit surface area is proportional to the fourth power of thermodynamic temperature.
Determine the temperature of the shell if the station is enveloped by a thin spherical black screen of nearly the same radius as the radius of the shell.
