MEDIUM
Earn 100
What are ions? How are positive and negative ions named?
Important Questions on Language of Chemistry
MEDIUM
EASY
EASY
Define the following.
Radical
EASY
Write the symbols and valencies of the following radicals:
Magnesium ion.
MEDIUM
EASY
HARD
Write the valency of the following radicals and represent them by a putting a positive or a negative sign.
MEDIUM
EASY
EASY
Write the symbols and valencies of the following radicals:
Bisulphate.
EASY
EASY
MEDIUM
HARD
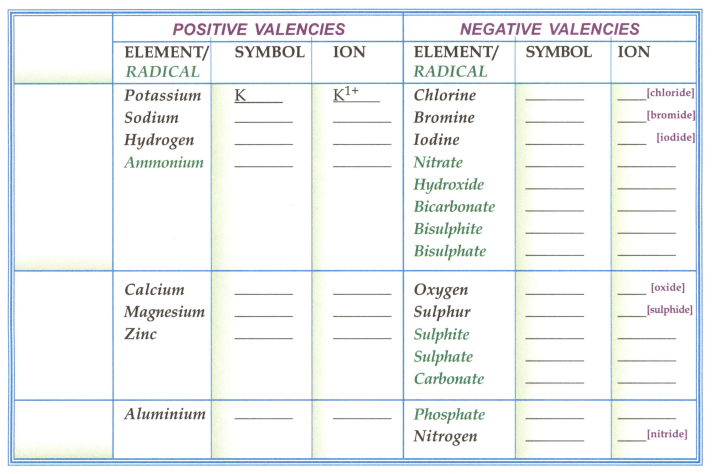
Fill in the symbol & ion [symbol with valency]
EASY
EASY
EASY
EASY
Match the following:-
| Column I | Column II | |
| (Elements) | (Symbol) | |
| (i) | Acidic Radicals | |
| (ii) | Basic Radicals | |
| (iii) | Monovalent Radicals | |
| (iv) | Variable Valency | |
| (v) | Tetravalent elements |
EASY
Write the symbols and valencies of the following compounds:
Aluminium ion.

