MEDIUM
Earn 100
What are simple buffers?
Important Questions on Equilibria
HARD
MEDIUM
EASY
MEDIUM
HARD
The pH of the solution after Expt. 2 is
MEDIUM
[Given:
MEDIUM
Which of the following mixtures will have the lowest pH at
EASY
HARD
A solution of weak base is titrated with of a strong acid . The variation of of the solution with the volume of added is shown in the figure below. What is the of the base? The neutralisation reaction is given by .
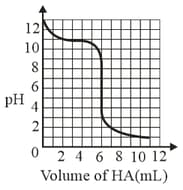
MEDIUM
EASY
HARD
MEDIUM
EASY
EASY
EASY
MEDIUM
HARD
HARD
[Given log 7 = 0.845 and Anti log (0.455) = 3.5]

