MEDIUM
Earn 100
What are the characteristics of an atom?
Important Questions on Chemical Bonding
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
In the given electron dot structure, the formal charge on each nitrogen atom (respectively) from left to right is _______
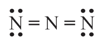
EASY
MEDIUM
EASY
MEDIUM

