EASY
Earn 100
What are the limitations of Newlands' law of octave?
(a)The law of octaves was applicable only up to calcium.
(b)The elements which were discovered after newland's octave did not follow the law of octave.
(c)Iron, which resembles cobalt and nickel in properties, was placed far away from these elements.
(d)All of the above
50% studentsanswered this correctly
Important Questions on The Periodic Table
EASY
EASY
EASY
MEDIUM
HARD
MEDIUM
MEDIUM
Chlorine, Bromine, and Iodine form a Dobereiner's Triad. If the approximate atomic mass of Chlorine is 35 and that of Iodine is 127:
Find the atomic mass of Bromine.
MEDIUM
In Dobereiner's triads , the atomic masses of lithium and potassium are and respectively, then what will be the atomic mass of sodium.
MEDIUM
HARD
Give one example of such a set of elements.
MEDIUM
HARD
(ii) Name the scientist who framed the above law.
(iii) Two elements A and B obey the law of octaves. How many elements are in between A and B?
HARD
(a)
(b)
Atomic mass of
Explain by giving a reason.
MEDIUM
HARD
(ii) What were the reasons for rejecting this classification?
MEDIUM
HARD
(ii) What were the reasons for rejecting the law of octaves?
HARD
Rearrange the columns and to match with the column .
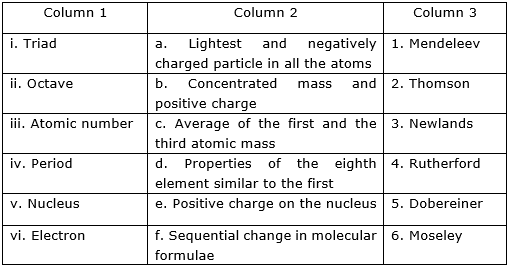
MEDIUM
MEDIUM

