HARD
Earn 100
What are the uses of methanol?
Important Questions on Alcohols, Phenols and Ethers
MEDIUM
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
Which of the following would give iodoform test?
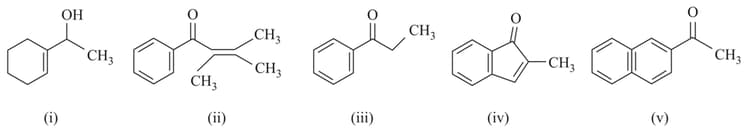
EASY
MEDIUM
EASY
EASY
MEDIUM
HARD
EASY
EASY
EASY
HARD
(a) What are X, Y and Z?
(b) Write a balanced chemical equation of the reaction which takes place when X and Y combine to form Z. Indicate the conditions under which the reaction occurs.
EASY
EASY

