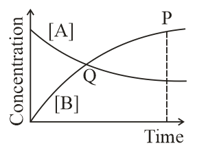
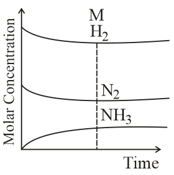
What do you mean by dynamic equilibrium?
Important Questions on Equilibrium
Which of the following statement is correct about chemical equilibrium?
(i) At equilibrium both the reactants and products coexist.
(ii) At equilibrium rate of forward reaction is greater than the rate of backward reaction.
Graph of a reversible process;
is given. Analyse the graph and answer the following question.
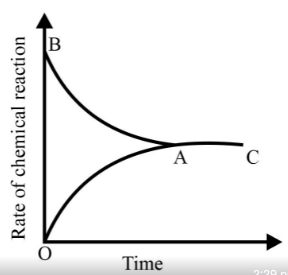
Identify the part of the graph which represents the equilibrium state?
At equilibrium, the mass of each of the reactants and products remains constant.
At equilibrium, the rate of forward reaction is equal to the rate of backward reaction.
Graph of a reversible process;
is given. Analyse the graph and answer the following question.
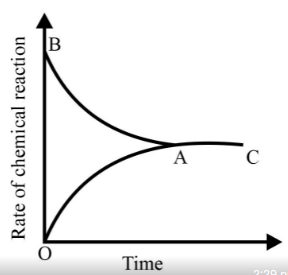
Identify the part of the graph which represents the forward reaction
[ OA, BA, AC]
Graph of a reversible process,
is given. Analyse the graph and answer the following question.
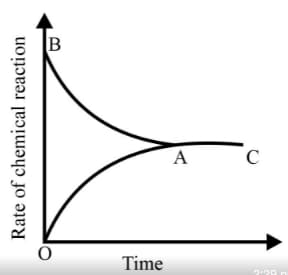
From the given statements, select the correct ones regarding chemical equilibrium.
(i0 The chemical equilibrium is 'static' at the molecular level.
(ii) Both reactants and products co-exist.
(iii) The rates of forward reaction and backward reactions are equal.
(iv) Chemical equilibrium is attained in an open system.
Using the data provided, find the value of equilibrium constant for the following reaction at and atm pressure.
Among the following points indicated on the graph, which one represents equilibrium?
Kp for the reaction
is at certain temperature. What lowest relative humidity of air can be achieved using as drying agent at that temperature ?
Aqueous tension at the given temperature = 16 torr
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
If and are equilibrium constants for the reactions and , respectively, then find out the equilibrium constant for the reaction .