What do you mean by qualitative analysis in chemistry?
Important Questions on Analytical Chemistry
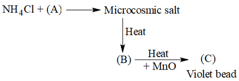
In the above given reaction reactant (A) and product (B) and (C) are :
An aqueous solution of an unknown compound gives the following reactions.
(i) It gives brown precipitate with alkaline solution
(ii) It forms & evolved when reacts with gas.
(iii) It liberates from an acidified solution.
(iv) It gives orange yellow colour with acidified titanic sulphate solution.
Identify and give the chemical equations for the reactions (i), (ii) & (iii).
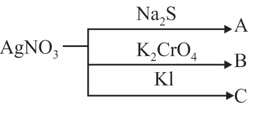
The colour of ppt. and respectively is
Identify the salt 'Q' from the following observation:
When ammonium hydroxide is added to salt 'Q', a white gelatinous precipitate with formation of are obtained that is soluble in excess of .
A metal chloride shows the following reactions:
(i) When is passes in an acidified aqueous solution of a black ppt is a obtained.
(ii) The precipitate obtained in step (i) is not soluble in yellow ammonium sulphide.
(iii) When a solution of stannous chloride is added to an aqueous solution of ,a white precipitate is obtained which turns grey on addition of more of stannous chloride.
(iv) When an aqueous solution of is added to an aqueous solution of , a red precipitate is obtained which dissolves on addition of excess of .
Identify and write down the equations for the reaction at steps (i), (iii) & (iv).

