What factors affect the reaction rates? Which condition in reversible reactions at equilibrium will maximize yield?
Important Questions on How Can We Shift the Balance of a Reaction?
The flow diagram refers to processes used in the manufacture of important compounds containing nitrogen.
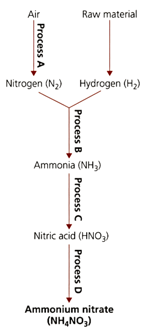
State the names of processes and .
The flow diagram refers to processes used in the manufacture of important nitrogen containing compounds.
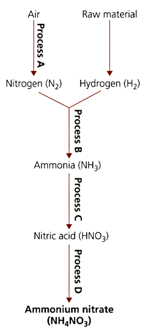
Identify one source of hydrogen for process .
The flow diagram refers to processes used in the manufacture of important nitrogen containing compounds.
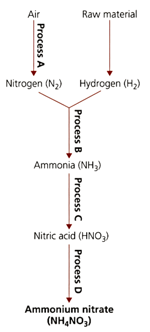
Suggest how the increase in temperature could affect process .
The flow diagram refers to processes used in the manufacture of important nitrogen containing compounds.
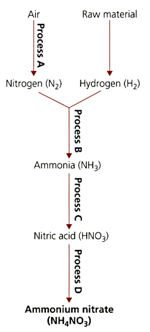
Suggest how the decrease in pressure could affect process .
Read the information below to identify the features of the experiment.
Sandstone contains sand (mainly silicon dioxide) and calcium carbonate. Excess sandstone was reacted with dilute hydrochloric acid.
. The rate of the reaction was measured during the mass lost during the reaction.
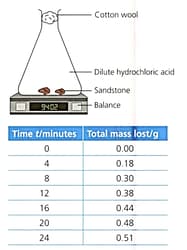
Identify the variables in this experiment and suggest how they were controlled.
Read the information below to identify the features of the experiment.
Sandstone contains sand (mainly silicon dioxide) and calcium carbonate. Excess sandstone was reacted with dilute hydrochloric acid. . The rate of the reaction was measured during the mass lost during the reaction.
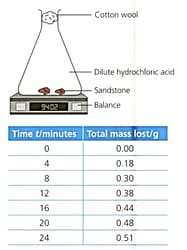
Outline, using idea about particles colliding, how the rate of reaction changed during the experiment.
When carbon monoxide and steam are passed over a heated iron catalyst they react as follows:
The forward reaction is exothermic and backward reaction is endothermic. Predict, using the information about the experiment, the effect on the yield of hydrogen caused by increasing the quantity of steam.
