MEDIUM
Earn 100
What happens when calcium reacts with water?
Important Questions on Sorting Materials into Groups
EASY
EASY
MEDIUM
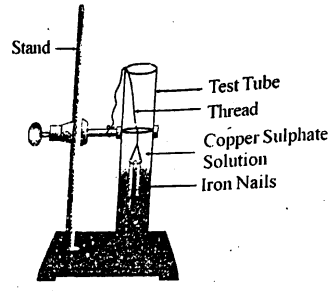
Write a chemical equation for the reaction taking place in the test tube. How does the colour of the solution change? What is the change in colour of iron nails?
EASY
EASY
Statement I: Sodium metal reacts violently with water to produce heat and fire.
Statement II: Potassium metal reacts violently with water to form potassium hydroxide and hydrogen gas.
Select the correct answer from the option given below
MEDIUM
EASY
EASY
EASY
EASY
EASY
MEDIUM
HARD
(a) potassium
(b) sodium
(c) calcium
HARD
EASY
HARD
HARD
HARD
(a) What is metal M?
(b) What is solution S? Is it acidic or alkaline?
(c) What is gas G?
(d) Write a balanced chemical equation for the reaction which takes place when metal M reacts with water.
(e) Is this reaction exothermic or endothermic?
EASY
HARD

