What happens when concentrated hydrochloric acid is heated with manganese dioxide?
Important Questions on Study of Compounds - Hydrogen Chloride
Distinguish between the following pair of compounds using the reagent given in the bracket.
Dilute hydrochloric acid and dilute sulphuric acid. (using lead nitrate solution)
Choose the method of preparation of Lead chloride, from the methods given in the list:
[List: A. Neutralization B.Precipitation C.Direct combination D.Substitution]
Manganese dioxide and copper(II) oxide. (using concentrated )
Copper oxide reacts with hydrochloric acid.
Hydrogen chloride is a neutral gas and dissolves in water to form an acidic solution.
Describe how sodium carbonate can be used to confirm that an aqueous solution contains an acid.
The acid on mixing with silver nitrate solution produces a white precipitate which is soluble in excess ammonium hydroxide.
Account for the following:
Dilute hydrochloric acid cannot be concentrated by distilling the dilute acid.
(i) Iron
(ii) Sodium sulphite
Action of dilute hydrochloric acid on iron (II) sulphide.
The action of dilute hydrochloric acid on sodium sulphide.
Name the following:
A compound which is insoluble in cold water but soluble in excess of ammonium hydroxide.
Choose from the following list, as to what matches the description given below:
[hydrogen, carbon dioxide gas, silver chloride, , chlorine, fountain experiment, lead nitrate, sulphur, sodium sulphite, sodium nitrate]
A gas liberated when calcium hydrogen carbonate is treated with dilute hydrochloric acid.
Account for the following:
When a sample of solid sodium hydroxide which has been left exposed to air for some time is added to dilute hydrochloric acid, a brisk effervescence is noted.
Each of the gases, sulphur dioxide, hydrogen sulphide and chlorine can be obtained by the reaction of hydrochloric acid with a suitable reagent. For each of these gases, write a chemical equation for its formation by using hydrochloric acid.
Action of dilute hydrochloric acid on magnesium sulphite.
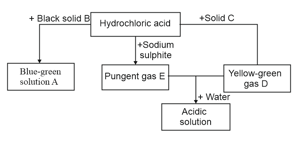
Name the products formed when aqueous sodium hydroxide is added to solution .

