EASY
Earn 100
What happens when dilute sulphuric acid is added to a salt containing sulphide radical and heated?
Important Questions on Practical Chemistry
MEDIUM
side products
side products
side products
The sum of the total number of atoms in one molecule each of and is ________
HARD
Distinguish between the following pair of compounds using a reagent as a chemical test:
- Magnesium chloride and magnesium nitrate solution.
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
Write chemical equation for the following:
Nitrogen dioxide is formed from a metallic nitrate.
MEDIUM
(Dilute hydrochloric acid, dilute nitric acid and dilute sulphuric acid)
Which acid will give a precipitate with barium chloride solution?
MEDIUM
Some reactions are given for salt(X).
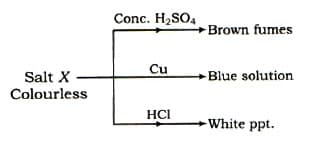
Which of the following salt can be satisfied all the conditions?
EASY
HARD
To an acid solution of an anion a few drops of KMnO4 solution are added. Which of the following, if present will not decolourise the KMnO4 solution
HARD
MEDIUM
EASY
The chemical formula of A and B are respectively :
HARD
HARD
The solution will :
MEDIUM
MEDIUM

