What happens when we put a burning wood in a jar containing hydrogen chloride gas.
Important Questions on Study of Compounds - Hydrogen Chloride
State one relevant reason for the following:
- Hydrogen chloride gas fumes in moist air.
Assertion (A): gas is dried by passing through concentrated .
Reason (R): gas reacts with that gives white fumes
Choose the method of preparation of Lead chloride, from the methods given in the list:
[List: A. Neutralization B.Precipitation C.Direct combination D.Substitution]
In which form of complex, platinum is dissolved in aqua regia?
For the preparation of hydrochloric acid in the laboratory, what arrangement is done to dissolve hydrogen chloride gas in water?
Distinguish between the following pair of compounds using the reagent given in the bracket.
Dilute hydrochloric acid and dilute sulphuric acid. (using lead nitrate solution)
Manganese dioxide and copper(II) oxide. (using concentrated )
Why is such an arrangement necessary? Give two reasons.
Hydrogen chloride is a neutral gas and dissolves in water to form an acidic solution. Explain why dry hydrogen chloride gas is neutral.
Name the gas evolved when the following mixtures are heated:
Manganese dioxide and concentrated hydrochloric acid.
Account for the following:
When a sample of solid sodium hydroxide which has been left exposed to air for some time is added to dilute hydrochloric acid, a brisk effervescence is noted.
Action of dilute hydrochloric acid on magnesium sulphite.
The action of dilute hydrochloric acid on sodium sulphide.
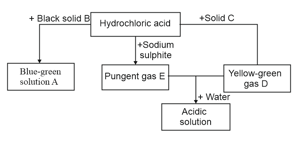
Name the products formed when aqueous sodium hydroxide is added to solution .
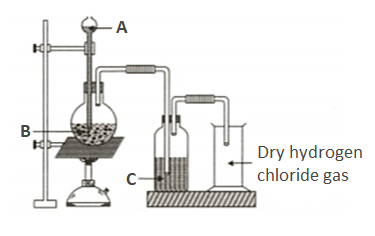
Name the substance that is added from the thistle funnel.

