What is Regelation?
Important Questions on Thermal Properties of Matter
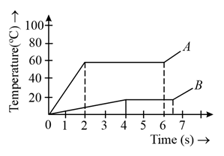
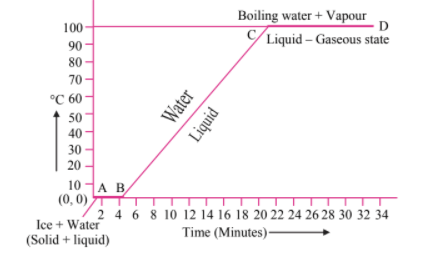
Explain the following temperature versus time graph:
A thermally insulated cubical box of side length and wall thickness containing of ice is closed on all sides. The mass of ice melted in hours is (Thermal conductivity of the material of the box latent heat of ice and ambient temperature )
[ Specific heat of water Latent heat of water ]
A steam engine intakes of steam at per minute and cools it down to . If latent heat of vaporization of steam is , then the heat rejected by the steam engine per minute is _____
(Given : specific heat capacity of water : )
Heat required to melt of ice is . A man melts of ice by chewing in one minute. His power is______
[Take specific heat of water and latent heat of steam
(Latent heat of ice is and )
Two substances and of equal mass are heated at a uniform rate of under similar conditions. A graph between temperature and time is shown in the figure. The ratio of heat absorbed by them during fusion is