EASY
Earn 100
What is calomel electrode?
Important Questions on Electrochemistry
HARD
The of different types of half cells are as follows :
(Where is the electromotive force). Which of the above half cells would be preferred to be used as reference electrode
MEDIUM
HARD
EASY
MEDIUM
Some materials are given below:
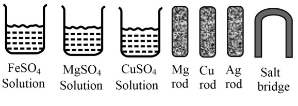
Write the chemical equation fo the reaction taking place at the cathode.
EASY
EASY
EASY
EASY
EASY
MEDIUM
Some materials are given below:
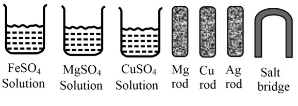
Which is the anode of this cell?
HARD
MEDIUM
An article made of copper is to be electroplated with silver.
What should be the cathode and the anode made up of?
MEDIUM
Depict the galvanic cell in which the reaction
takes place. Further show:
Which of the electrode is negatively charged?
EASY
Cations migrate during electrolysis.
EASY
MEDIUM
MEDIUM
EASY
MEDIUM

