What is meant by the rate controlling step in a reaction?
Important Questions on Chemical Kinetics
Consider the following reaction
The rate can be expressed as
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

The reaction takes place through the mechanism given below
The overall order of the reaction is _____.
(Assume that all these gases behave as ideal gases)
For a reaction
The proposed mechanism is as given below:
1. (slow)
2. (fast)
Out of steps (1) and (2), which one is rate-determining step?
For a reaction,
The rate law, is Rate, . Can this reaction be an elementary reaction?
Which one of the following statements is correct?
The reaction rate for the reaction
was measured as a function of concentrations of different species. It was observed that
where square brackets are used to denote molar concentrations. The equilibrium constant
(Nearest integer)
Value of (question is modified.)
For an elementary chemical reaction, , the expression for is:
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
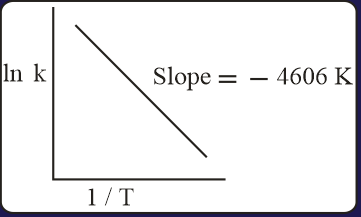
The activation energy of the backward reaction exceeds that of the forward reaction by (in ). If the pre-exponential factor of the forward reaction is times that of the reverse reaction, the absolute value of for the reaction at is ____.
(Given; and is the Gibbs energy)
For a reaction rate law expression is :
Rate = .
Can the reaction be an elementary ? Explain.
The reaction is an elementary reaction.
For a certain quantity of reactants, if the volume of the reaction vessel is reduced by a factor of the rate of the reaction increases by a factor of _____ . (Round off to the Nearest Integer).

