EASY
12th Uttar Pradesh Board
IMPORTANT
Earn 100
What is photo-electric effect?

Important Questions on Dual Nature of Radiation and Matter
EASY
12th Uttar Pradesh Board
IMPORTANT
For a certain metal threshold wavelength is . Calculate the work function of the metal.
HARD
12th Uttar Pradesh Board
IMPORTANT
When a metallic surface is illuminated with light of frequency the maximum kinetic energy of emitted electrons is . When the same surface is illuminated by light of frequency the maximum kinetic energy of emitted electrons is . Find out work function of the metal.
MEDIUM
12th Uttar Pradesh Board
IMPORTANT
The frequency of a photon of frequency f after falling through height H in the uniform gravitational field due to earth is . Assume that photon manifest any increase in energy by changing frequency. Choose the correct relation : (Take acceleration due to gravity g)
HARD
12th Uttar Pradesh Board
IMPORTANT
According to Einstein’s photoelectric equation, the plot of the kinetic energy of the emitted photo electrons from a metal versus the frequency, of the incident radiation gives a straight line whose slope
EASY
12th Uttar Pradesh Board
IMPORTANT
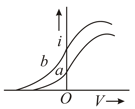
Figure shows the variation of photoelectric current with voltage between the electrodes in a photo-cell for two different radiations. If and are the intensities of the incident radiations and and are the respective frequencies, then
MEDIUM
12th Uttar Pradesh Board
IMPORTANT
A beam of light of intensity is normally incident on a plate of area . If plate absorbs fraction of incident light and reflects rest of it back, then the force exerted by the incident light on the plate is given by [=speed of light]
MEDIUM
12th Uttar Pradesh Board
IMPORTANT
STATEMENT-1: The relative speed of separation between two photons travelling in opposite direction in vacuum is zero, where .
STATEMENT-2: Speed of a photon in vacuum does not depend on the frame of reference.
MEDIUM
12th Uttar Pradesh Board
IMPORTANT
Light of wavelength falls on a metal surface of work function . Find the kinetic energy of photoelectrons. Take:
