HARD
Earn 100
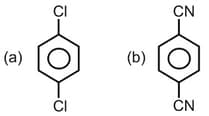
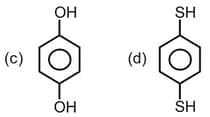
What is the covalent character if the bond length and dipole moment of are and respectively?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
The boiling point (in ) of cis but-2-ene and dipole moment (in ) of trans but- 2 -ene are respectively
EASY
Which of the following molecules has permanent dipole moment?
EASY
Which of the following compounds has zero dipole moment ?
HARD
Molecule AB has a bond length of and a dipole moment of 0.38 D. The fractional charge on each atom(absolute magnitude) is:
EASY
The dipole moments of and are in the order
EASY
If the dipole moment of toluene and nitro-benzene are and then what is the expected dipole moment of nitro toluene ?
EASY
If is the magnitude of charge and is the distance between the centres of positive and negative charges then dipole moment is given by
EASY
Which of the following has highest dipole moment?
MEDIUM
Which of the following possess net dipole moment?
MEDIUM
Which of the following set of molecules will have zero dipole moment?
EASY
Which compounds exhibits maximum dipole moment among the following?
MEDIUM
Each of the following options contains a set of four molecules. Identify the option(s) where all four molecules possess permanent dipole moment at room temperature.
EASY
Pick up the incorrect statement
MEDIUM
Dipole moment order of which of the following pairs of molecules is not conect?
EASY
Among the following, the most polar molecule is :
EASY
is a polar solvent while is a nonpolar solvent because has
HARD
The dipole moment of which of the following molecule is greater than zero?
HARD
Which substance has a dipole moment?
EASY
Which of the following molecules has the maximum dipole moment?
HARD
For which of the following molecule significant ?