What is the atomic number and mass number of the element whose atomic structure is shown in below?
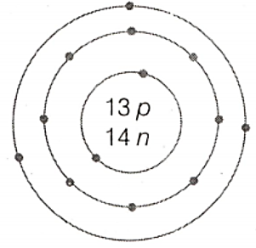

Important Questions on Structure of the Atom
In the Thomson's model of atom, which of the following statements are correct?
(i) The mass of the atom is assumed to be uniformly distributed over the atom.
(ii) The positive charge is assumed to be uniformly distributed over the atom.
(iii) The electrons are uniformly distributed in the positively charged sphere.
(iv) The electrons attract each other to stabilise the atom.
The following diagram depicts the Rutherford’s experiment.
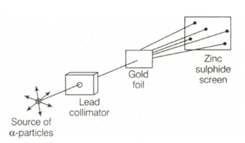
Atomic models have been improved over the years. Arrange the following atomic models in the order of their chronological order.
(i) Rutherford's atomic model
(ii) Thomson's atomic model
(iii) Bohr's atomic model.
Which of the following are true for an element?
(i )Atomic number Number of protons number of electrons
(ii) Mass number Number of protons number of neutrons
(iii) Atomic mass Number of protons Number of neutrons
(iv) Atomic number Number of protons Number of electrons.
Different isotopes are matched with their uses as
(i) -To treat cancer
(ii) -To produce electricity
(iii) -To treat goitre
(iv) -In agricultural research
Which of the above matches are correct?
