EASY
Earn 100
What is the atomicity of chlorine and argon?
(a)Monatomic and diatomic
(b)Monatomic and tetraatomic
(c)Diatomic and monatomic
(d)Diatomic and polyatomic
50% studentsanswered this correctly
Important Questions on Kinetic Theory
EASY
EASY
EASY
Given below are two statements:
Statement I: In a diatomic molecule, the rotational energy at a given temperature obeys Maxwell's distribution.
Statement II : In a diatomic molecule, the rotational energy at a given temperature equals the translational kinetic energy for each molecule.
In the light of the above statements, choose the correct answer from the options given below:
EASY
EASY
EASY
EASY
EASY
EASY
EASY
(R-Universal gas constant)
EASY
EASY
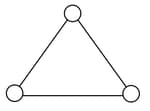
Consider a gas of triatomic molecules. The molecules are assumed to be triangular and made of massless rigid rods whose vertices are occupied by atoms. The internal energy of a mole of the gas at temperature T is:
EASY
EASY
EASY
a) Temperature of a gas is a measure of average kinetic energy of a molecule
b) Temperature of a gas depends on the nature of the gas
c) Heavier molecule has lower average speed
d) Lighter molecule has lower average speed
MEDIUM
EASY
EASY
EASY
Match the ratio for ideal gases with different type of molecules:
| Molecule Type | |
| (A) Monoatomic | (I) |
| (B) Diatomic rigid molecules | (II) |
| (C) Diatomic non-rigid molecules | (III) |
| (D) Triatomic rigid molecules | (IV) |
EASY

