EASY
JEE Main/Advance
IMPORTANT
Earn 100
What is the change in internal energy , for a system that does joules of work as it absorbs joules of heat?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
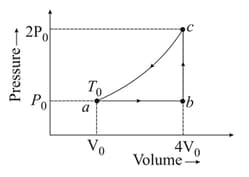
One mole of an ideal monoatomic gas is made to go through the cycle as shown in figure. Then the change in the internal energy in expanding the gas from to along the path is