EASY
Earn 100
What is the correct graph from the following? If during electrolysis the amount of product liberated at electrode is plotted against charge in Faraday.
(a)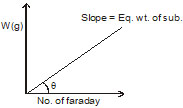

(b)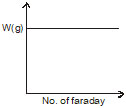

(c)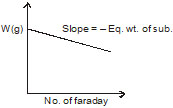

(d)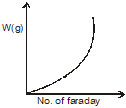

(e)None of these
50% studentsanswered this correctly
Important Questions on Electrochemistry
MEDIUM
EASY
HARD
MEDIUM
EASY
MEDIUM
MEDIUM
A current of flows for through an electrolytic cell containing a molten salt of metal . This results in the decomposition of of metal at the cathode. The oxidation state of in the molten salt is:
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
HARD
(Assume of electrons and molecular weight of as )
EASY
EASY

