What is the internal energy for an isothermal process.
Important Questions on Thermodynamics
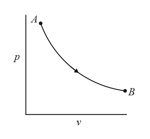
A sample of an ideal gas undergoes an isothermal process as shown by the curve in the diagram. If and represent the amount of heat absorbed the change in internal energy and the work done respectively, then which of the following statement is correct?
(Take , where is gas constant)
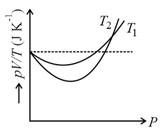
The figure shows the graph of versus for of hydrogen gas at two different temperatures, where and represents pressure, volume and temperature respectively.Then, the value of where the curve meet on the vertical axis, is
| Column – 1 | Column – 2 | Column – 3 |
| (I) | (i) Isothermal | (P) 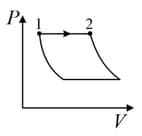 |
| (II) | (ii) Isochoric | (Q) 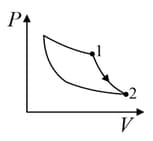 |
| (III) | (iii) Isobaric | (R) 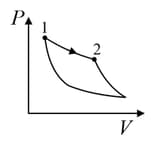 |
| (IV) | (iv) Adiabatic | (S) 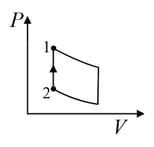 |
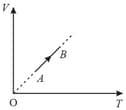
Consider the following thermodynamical variables
(i) Pressure
(ii) Internal Energy
(iii) Volume
(iv) Temperature
Out of these, the intensive variable(s) is (are)
| Column – 1 | Column – 2 | Column – 3 |
| (I) | (i) Isothermal | (P) 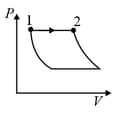 |
| (II) | (ii) Isochoric | (Q) 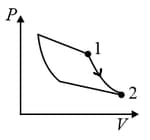 |
| (III) | (iii) Isobaric | (R) 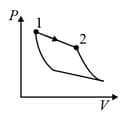 |
| (IV) | (iv) Adiabatic | (S) 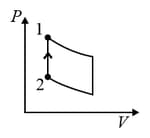 |
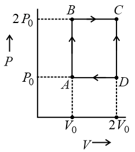
A system goes from to via two processes and shown in the figure. If and are the changes in internal energies in the processes I and Il respectively
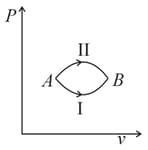
If the indicator diagram for expansion of gas is as shown, the gas
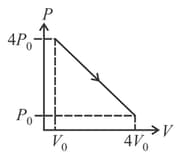
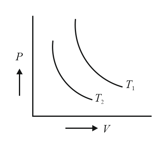
Two isothermals are shown in figure at temperature and . Which of the following relations is correct?