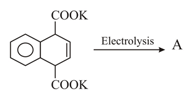
MEDIUM
Earn 100
What is the mechanism behind reduction of carboxylic acids to alcohol?
Important Questions on Aldehydes, Ketones and Carboxylic Acids
MEDIUM
chlorosodium acetate on boiling with aqueous sodium nitrite gives
HARD
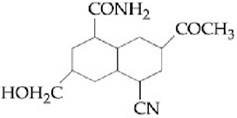
In the reaction,
,
the product C is :
HARD
The major product of the following reaction is:
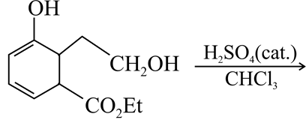
EASY
The correct structure of the product in the following reaction is
EASY
In the presence of a small amount of phosphorous, aliphatic carboxylic acid reacts with chlorine or bromine to yield a reaction in which, hydrogen is been replaced by halogen. This reaction is known as
MEDIUM
Propanoic acid undergoes reaction to give chloropropanoic acid. The product obtained is
MEDIUM
The major product of the following reaction is:
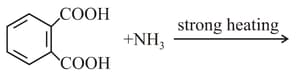
EASY
Why benzoic acid does not undergo Friedel-crafts reaction?
MEDIUM
How will you bring about the following conversion in not more than two steps?
Benzoic acid to m-Nitrobenzyl alcohol
MEDIUM
Which of the following derivatives of alcohols is unstable in an aqueous base?
EASY
Give the chemical equation for the reaction of with following:
MEDIUM
Which of the following acid will form an (a) Anhydride on heating and (b) Acid imide on strong heating with ammonia?
HARD
The most suitable reagent for the given conversion is:
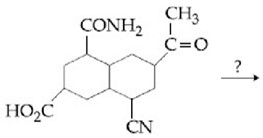
EASY
Which of the following acids, does NOT undergo Hell-Volhard-Zelinsky reaction?
EASY
Name the end product in the following series of reactions ections
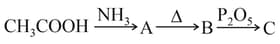
MEDIUM
The major product of nitration of benzoic acid is
MEDIUM
The major product obtained in the reaction of aniline with acetic anhydride is
MEDIUM
An organic compound A upon reacting with gives B. On heating, B gives C. C in presence of KOH reacts with Br2 to give CH3CH2NH2. A is :
HARD
With dehydrating agent present which dicarboxylic acid is least reactive towards forming an anhydride?