MEDIUM
Earn 100
What is the nature of the pressure-volume graph in Boyle's law:
(a)Straight line
(b)Circle
(c)Elliptical
(d)Rectangular hyperbola
50% studentsanswered this correctly
Important Questions on Gaseous State
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
For the given isotherms, which of the following is correct for
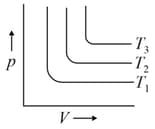
EASY
EASY
MEDIUM
EASY
EASY
MEDIUM
[Gas constant, ]
EASY
MEDIUM
MEDIUM
Choose the correct option for graphical representation of Boyle's law, which shows a graph of pressure vs. volume of a gas at different temperatures:
MEDIUM
EASY
EASY
EASY
EASY
At constant pressure, the volume of a fixed mass of a gas varies as a function on temperature as shown in the graph
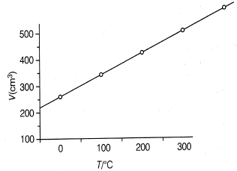
The volume of the gas at is larger than that at by a factor of

