EASY
Earn 100
What is the order for the following reactions?
, rate
Important Questions on Chemical Kinetics
EASY
MEDIUM
EASY
EASY
HARD
EASY
HARD
What will be the order of the reaction for hydrolysis of methyl acetate with by using the data provided?
Time
Volume of acid
EASY
MEDIUM
For the chemical reaction
, the reaction proceeds as follows
(Fast)
, (Slow)
the rate law expression should be given as
EASY
MEDIUM
EASY
EASY
EASY
EASY
MEDIUM
EASY
EASY
HARD
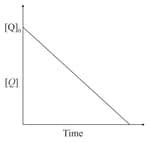
the time taken for reaction of is twice the time taken for reaction of . The concentration of varies with reaction time as shown in the figure.The overall order of the reaction is: