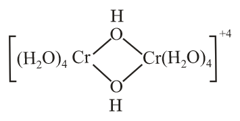
What is the oxidation number of chromium in the dimeric hydroxo bridged species?


Important Questions on Coordination Compounds
Give the name describing the type of structural isomerism displayed by each of the following pairs;
and
Give the name describing the type of structural isomerism displayed by each of the following pairs;
and
Give the name describing the type of structural isomerism displayed by each of the following pairs;
and
Give the name describing the type of structural isomerism displayed by each of the following pairs,
and
exists in two isomeric forms '' and ''. Isomer '' reacts with to give white precipitate, but does not react with Isomer '' gives a white precipitate with , but does not react with . Identify '' and '' and write their structural formula.
exists in two isomeric forms '' and ''. Isomer '' reacts with to give white precipitate, but does not react with Isomer '' gives a white precipitate with , but does not react with .
Name the type of isomerism involved.
