EASY
Earn 100
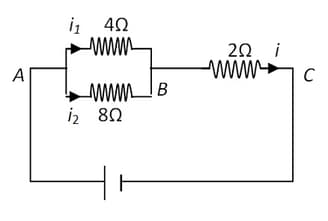
What is the practical application of the electrolysis of copper sulphate solution? Briefly, describe one such application?
Important Questions on Redox Processes (AHL)
EASY
MEDIUM
State one relevant observation for of the following:
At the anode, when molten lead bromide is electrolyzed using graphite electrodes.
EASY
HARD
State one relevant reason for the following:
- Graphite anode is preferred to platinum in the electrolysis of molten lead bromide.
MEDIUM
MEDIUM
EASY
EASY
EASY
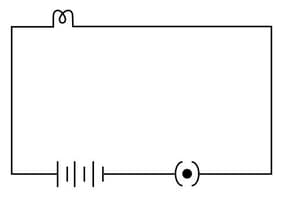
Will the bulb in the following circuits glow? Justify your answer.
EASY
What do you understand by a closed electric circuit?
EASY
EASY
EASY
EASY
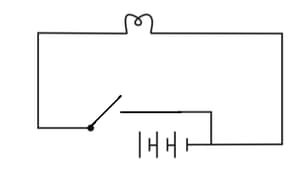
Will the bulb in the following circuits glow? Justify your answer.
EASY
EASY
EASY
MEDIUM
EASY
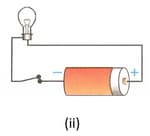
Figures below show a cell connected to a bulb through insulated copper wires. State with the reason in which case will the bulb (i) glow, and (ii) not glow.
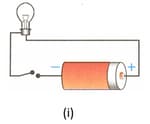
EASY