MEDIUM
Earn 100
What is the primary motive behind international business?
(a)To benefit from tax incentives
(b)To increase domestic sales
(c)To reduce competition
(d)To earn foreign exchange
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
HARD
MEDIUM
EASY
EASY
Match the physical quantities with their correct units.
| List-I | List-II | ||
| (A) | Planck's constant | (I) | |
| (B) | Torque | (II) | |
| (C) | Weight | (III) | |
| (D) | Specific volume | (IV) |
The correct match is
EASY
EASY
EASY
EASY
Starting with the same initial conditions, an ideal gas expands from volume to in three different ways. The work done by the gas is if the process is purely isothermal, if purely isobaric and purely adiabatic. Then
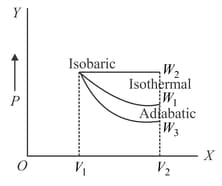
EASY
EASY
EASY
EASY
EASY
kJ/mol; will be
MEDIUM
EASY
MEDIUM
EASY
EASY
EASY
An ideal gas is taken around the cycle as shown in the diagram
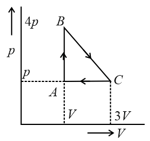
The total work done by the gas during the cycle is

