MEDIUM
Earn 100
What is the reactant [X] which produces cyclohexanone and formaldehyde on reaction with HIO4 ?
(a)Cyclohexan, 1, 2-diol
(b)Cyclohexan, 1, 2, 3-triol
(c)1-hydroxy cyclohexyl methanol
(d)2-hydroxy cyclohexyl methanol
50% studentsanswered this correctly
Important Questions on Alcohols, Phenols and Ethers
HARD
Two compounds and with same molecular formula undergo Grignard reaction with methylmagnesium bromide to give products and . Products and show following chemical tests.
| Test | C | D |
| Ceric ammonium nitrate Test | Positive | Positive |
| Lucas Test | Turbidity obtained after five minutes | Turbidity obtained Immediately |
| Iodoform Test | Positive | Negative |
and respectively are
MEDIUM
The major product of the following reaction is
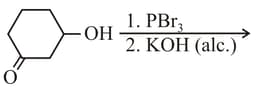
EASY
HARD
Identify and in the following reactions
EASY
EASY
Identify in the following series of reaction
HARD
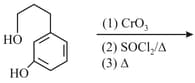
MEDIUM
The reactant ' in the following reaction is
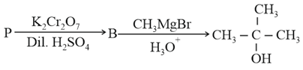
MEDIUM
EASY
MEDIUM
HARD
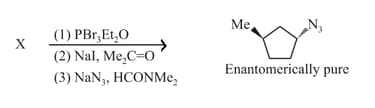
MEDIUM
EASY
Which of following alcohols gives white turbidity almost immediately with the Lucas reagent at room temperature?
(i) -Butanol
(ii) tertiary-Butanol
(iii) Benzyl alcohol
(iv) Allylic alcohol
EASY
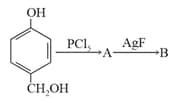
What is B in the above scheme?
MEDIUM
Identify A and B respectively in the following reaction:
MEDIUM
MEDIUM
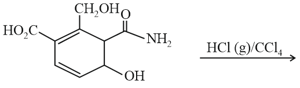
MEDIUM
EASY

