MEDIUM
Earn 100
What is the term used to describe the price at which the quantity demanded equals the quantity supplied?
(a)Equilibrium price
(b)Market price
(c)Floor price
(d)Ceiling price
50% studentsanswered this correctly
Important Questions on Equilibrium
HARD
For the given reaction, if the initial pressure is and the pressure at time is at a constant temperature and constant volume . The fraction of decomposed under these conditions is . The value of is (nearest integer)
HARD
EASY
HARD
For a reaction at equilibrium
the relation between dissociation constant , degree of dissociation and equilibrium pressure is given by :
MEDIUM
EASY
observed pressure for the reaction mixture in equilibrium is at . What is the value of for the reaction?
EASY
MEDIUM
For the reaction
if the initial concentration of and moles of is consumed at equilibrium, the correct expression of is.
HARD
MEDIUM
HARD
MEDIUM
Which of the following statements is correct?
MEDIUM
EASY
EASY
EASY
MEDIUM
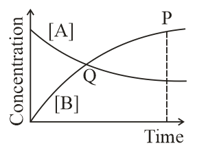
Among the following points indicated on the graph, which one represents equilibrium?
EASY
MEDIUM
MEDIUM
Which is the most effective dehydrating agent at ? (Aqueous tension at is atm)