MEDIUM
JEE Main
IMPORTANT
Earn 100
What is the work function of the metal if the light of wavelength generates photoelectrons of velocity from it?
(Mass of electron , velocity of light , Planck's constant , Charge of electron )
(a)
(b)
(c)
(d)
33.33% studentsanswered this correctly

Important Questions on Structure of Atom
EASY
JEE Main
IMPORTANT
Among the following, the energy of orbital is lowest in:
EASY
JEE Main
IMPORTANT
The group number, number of valence electrons, and valency of an element with atomic number respectively, are:
MEDIUM
JEE Main
IMPORTANT
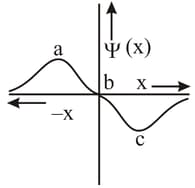
The electrons are more likely to be found:
MEDIUM
JEE Main
IMPORTANT
At temperature , the average kinetic energy of any particle is . The de Broglie wavelength follows the order:
MEDIUM
JEE Main
IMPORTANT
The ground state energy of a hydrogen atom is . The energy of second excited state of ion in is:
MEDIUM
JEE Main
IMPORTANT
Which of the graphs shown below does not represent the relationship between incident light and the electron ejected from metal surface?
MEDIUM
JEE Main
IMPORTANT
The ratio of the shortest wavelength of two spectral series of hydrogen spectrum is found to be about . The spectral series are:
EASY
JEE Main
IMPORTANT
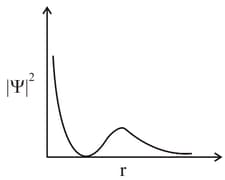
The graph between and (radical distance) is shown below. This represents: