What is viscosity?
Important Questions on States of Matter: Gaseous and Liquid States
Based on the given figure, the number of correct statement/s is/are
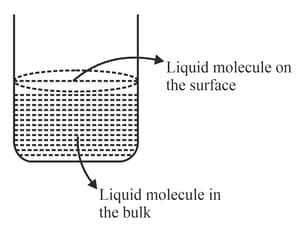
A. Surface tension is the outcome of equal attractive and repulsion forces acting on the liquid molecule in bulk.
B. Surface tension is due to uneven forces acting on the molecules present on the surface.
C. The molecule in the bulk can never come to the liquid surface.
D. The molecules on the surface are responsible for vapour pressure if the system is a closed system.
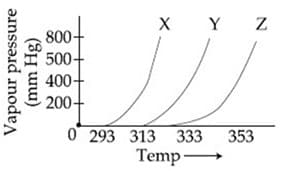
The following inferences are made:
has higher intermolecular interactions compared to
has lower intermolecular interactions compared to
has lower intermolecular interactions compared to
The correct inferences is/are:

In deriving Bernoulli’s equation, we equated the work done on the fluid in the tube to its change in the potential and kinetic energy. Do the dissipative forces become more important as the fluid velocity increases? Discuss qualitatively.
(Choose from: Blood/Honey/Water/Shampoo)
Glycerine flows steadily through a horizontal tube of length and radius . If the amount of glycerine collected per second at one end is , what is the pressure difference between the two ends of the tube? (Density of glycerine and viscosity of glycerine ). [You may also like to check if the assumption of laminar flow in the tube is correct].
(Choose from: Blood/Water/Emulsion paint)
What is the effect of pressure on the viscosity of gas?
The diagram shows a cup of tea seen from above. The tea has been stirred and is now rotating without turbulence. A graph showing the speed with which the liquid is crossing points at a distance