EASY
Earn 100
What kind of impurities increases surface tension?
Important Questions on Mechanical Properties of Fluids
EASY
A rectangular film of liquid is extended from to . If the work done is , the value of the surface tension of the liquid is
MEDIUM
The qualitative sketches I, II and III given below show the variation of surface tension with molar concentration of three different aqueous solutions of KCl, and at room temperature. The correct assignment of the sketches is -
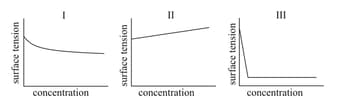
EASY
Three liquids of densities, and (with, , having the same value of surface tension, , rise to the same height in three identical capillaries. The angles of contact, and obey
EASY
When one end of the capillary is dipped in water, the height of the water column is . The upward force of due to surface tension is balanced by the force due to the weight of the water column. The inner circumference of the capillary is
(Surface tension of water
EASY
Water rises to a height 'h' in capillary tube. If the length of capillary tube above the surface of water is made less than 'h', then:
MEDIUM
A big water drop is formed by the combination of small water drops of equal radii. The ratio of the surface energy of drops to the surface energy of the big drop is
HARD
A small soap bubble of radius 4cm is trapped inside another bubble of radius 6cm without any contact. Let be the pressure inside the inner bubble and , the pressure outside the outer bubble. Radius of another bubble with pressure difference between its inside and outside would be:
EASY
A soap bubble having radius of is blown from a detergent solution having a surface tension of The pressure inside the bubble equals at a point below the free surface of water in a container. Taking density of water the value of is
HARD
Assume that a drop of a liquid evaporates by a decrease in its surface energy so that its temperature remains unchanged. The minimum radius of the drop for this to be possible is. (The surface tension is , the density of the liquid is and is its latent heat of vaporisation.)
HARD
If two glass plates have water between them and are separated by very small distance (see figure), it is very difficult to pull them apart. It is because the water in between forms cylindrical surface on the side that gives rise to lower pressure in the water in comparison to atmosphere. If the radius of the cylindrical surface is R and surface tension of water is T then the pressure in water between the plates is lower by:
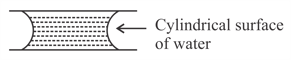
MEDIUM
A capillary tube is immersed vertically in water and the height of the water column is . When this arrangement is taken into a mine of depth d, the height of the water column is . If R is the radius of earth, the ratio is :
MEDIUM
A soap bubble, blown by a mechanical pump at the mouth of a tube increases in volume with time at a constant rate. The graph that correctly depicts the time dependence of pressure inside the bubble is given by:
HARD
A large number of liquid drops each of radius coalesce to form a single drop of the radius . The energy released in the process is converted into kinetic energy of the big drop so formed. The speed of the big drop is (given surface tension of the liquid , density )
MEDIUM
The following observations were taken for determining surface tension of water by capillary method:
diameter of capillary,
rise of water,
Using and the simplified relation the possible error in surface tension is closest to:
EASY
A glass capillary tube is of the shape of a truncated cone with an apex angle so that its two ends have cross-sections of different radii. When dipped in water vertically, the water rises in it to a height , where the radius of its cross-section is . If the surface tension of water is , its density is , and its contact angle with glass is , then the value of will be ( is the acceleration due to gravity)
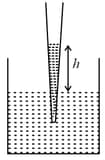
EASY
If is the mass of water that rises in a capillary tube of radius , then mass of water which will rise in a capillary tube of radius is:
MEDIUM
The ratio of surface tensions of mercury and water is given to be , while the ratio of their densities is . Their contact angles, with glass, are close to and , respectively. If it is observed that mercury gets depressed by an amount in a capillary tube of radius , while water rises by the same amount in a capillary tube of radius , then the ratio is close to
MEDIUM
Consider a bowl filled with water on which some black paper powder has been sprinkled uniformly. Now a drop of liquid soap is added at the center of the surface of the water. The picture of the surface immediately after this will look like
EASY
The radius of the soap bubble is doubled under isothermal condition. If be the surface tension of the soap bubble, the work done in doing so is given by
EASY
When a sparingly soluble substance like alcohol is dissolved in water, surface tension of water

