EASY
12th ICSE
IMPORTANT
Earn 100
What type of crystal defect is indicated in the diagram below?
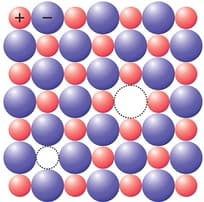

(a)Frenkel defect
(b)Schottky defect
(c)Interstitial defects
(d)
Frenkel and Schottky defects
50% studentsanswered this correctly

Important Questions on Solid State
EASY
12th ICSE
IMPORTANT
An ionic compound has a unit cell consisting of ion at the corners of a cube and ions on the centres of the faces of the cube. The empirical formula for this compound would be
EASY
12th ICSE
IMPORTANT
EASY
12th ICSE
IMPORTANT
In a compound, atoms of element form ccp lattice and those of element occupied of rd of tetrahedal voids. The formula at the compound will be
EASY
12th ICSE
IMPORTANT
crystallises in body centred cubic lattice with edge length, equal to . The distance between two oppositively charged ions in the lattice is
EASY
12th ICSE
IMPORTANT
EASY
12th ICSE
IMPORTANT
EASY
12th ICSE
IMPORTANT
EASY
12th ICSE
IMPORTANT
