EASY
Earn 100
What type of crystal defect is indicated in the diagram below ?
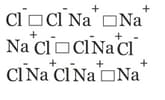
(a) Frenkel defect
(b)Schottky defect
(c)interstitial defect
(d)Frenkel and Schottky defects
50% studentsanswered this correctly
Important Questions on Solid State
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY
MEDIUM
) Frenkel defect is favored by small difference in the sizes of cation and anion.
) Frenkel defect is a metal excess defect.
) Trapping of electron in the lattice leads to formation of F-centres.
) Schottky defect has no effect on the physical property of solids.
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
Given below are two statements:
Statement I : Frenkel defects are vacancy as well as interstitial defects.
Statement II : Frenkel defect leads to colour in ionic solids due to presence of F-centres.
Choose the most appropriate answer for the statements from the options given below:
MEDIUM
EASY
EASY
EASY
EASY

