MEDIUM
Chemistry
IMPORTANT
Earn 100
What weight of must be decomposed to produce the sufficient quantity of carbon dioxide to convert of completely into . Atomic mass
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Mole Concept
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
If, used in this process is obtained through a process, in which of carbon is mixed with .
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
The number of molecules of the sweetener saccharin, which can be prepared
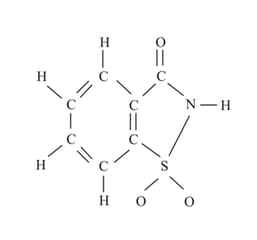
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
