MEDIUM
Earn 100
What will be the molarity of a saturated solution of ?
Important Questions on Ionic Equilibria
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
[Solubility product for ]
MEDIUM
HARD
EASY
HARD
MEDIUM
MEDIUM
Which of the following choices is correct for a mixture of and
HARD
[Given ]
MEDIUM
The stoichiometry and solubility product of a salt with the solubility curve given below is, respectively:
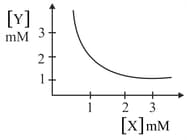
EASY
MEDIUM
EASY
If the solubility product of is , then the solubility of in pure water is _______
[Assuming that neither kind of ion reacts with water]
EASY
MEDIUM
MEDIUM
EASY

