EASY
Earn 100
What will be the products when an acid reacts with metals?
(a)Water and hydrogen gas
(b)Acid and hydrogen gas
(c)Salt and hydrogen gas
(d)Base and hydrogen gas
50% studentsanswered this correctly
Important Questions on Acids, Bases and Salts
MEDIUM
MEDIUM
MEDIUM
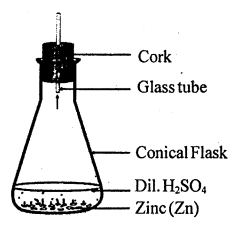
Write a chemical equation for the reaction taking place in the flask. Write name and one property of the gas evolved.
EASY
EASY
State one observation for the following:
A small piece of zinc is added to dilute hydrochloric acid.
MEDIUM
MEDIUM
MEDIUM
EASY
HARD
MEDIUM
EASY
MEDIUM
MEDIUM
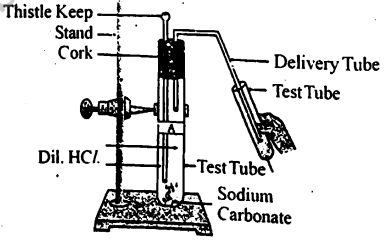
Which gas is produced during the reaction in the test tube? How does this gas react with calcium hydroxide/lime water?
EASY
EASY
EASY
MEDIUM
State one relevant observation for of the following:
Lead nitrate solution is mixed with dilute hydrochloric acid and heated.
MEDIUM
Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
MEDIUM

