EASY
NEET
IMPORTANT
Earn 100
What will be the total pressure of the gaseous mixture at equilibrium if is dissociated according to the following reaction, ( represents the equilibrium constant of the reaction)
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Equilibrium
EASY
NEET
IMPORTANT
For this reaction, If we start with mole of the compound, the total pressure at equilibrium would be
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
To which direction will the equilibrium shift?
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Consider the following graph for the chemical reaction
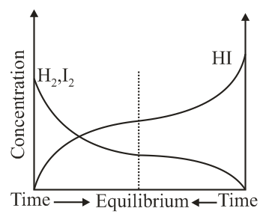
The inference that can be obtained from this graph about chemical equilibrium is that
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
