MEDIUM
JEE Main
IMPORTANT
Earn 100
When of oxygen gas is heated at constant pressure starting at , how much energy must be added to the gas as heat to triple its volume? (The molecules rotate but do not oscillate).
Important Questions on The Kinetic Theory of Gases
MEDIUM
JEE Main
IMPORTANT
The speeds of particles are as follows ( represents the number of particles that have speed of ):
| () |
What are (a) (b) and
(c)
MEDIUM
JEE Main
IMPORTANT
A gas is to be expanded from initial state to final state along either path or path on a diagram. Path consists of three steps: an isothermal expansion (work is in magnitude), an adiabatic expansion (work is in magnitude), and another isothermal expansion (work is in magnitude). Path consists of two steps: a pressure reduction at constant volume and an expansion at constant pressure. What is the change in the internal energy of the gas along path
MEDIUM
JEE Main
IMPORTANT
When was added as heat to a particular ideal gas, the volume of the gas changed from to while the pressure remained at . (a) By how much did the internal energy of the gas change? If the quantity of gas present was , find and (c) .
HARD
JEE Main
IMPORTANT
The mass of a gas molecule can be computed from its specific heat at constant volume (Note that this is not ). Take for neon and calculate (a) the mass of neon atom and (b) the molar mass of neon.
MEDIUM
JEE Main
IMPORTANT
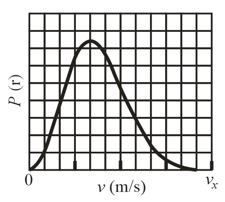
Given figure, gives the probability distribution for nitrogen gas. The scale of the horizontal axis is set by . What is the gas temperature and RMS speed of the molecules?
HARD
JEE Main
IMPORTANT
Suppose of an ideal diatomic gas, with molecular rotation but not oscillation, experiences a temperature increase of under constant-pressure conditions. What are the energy transferred as heat , (b) the change in internal energy of the gas, (c) the work done by the gas, and (d) the change in the total translational kinetic energy of the gas?
EASY
JEE Main
IMPORTANT
Find the rms speed of argon atoms at The molar mass of argon is .
MEDIUM
JEE Main
IMPORTANT
The temperature of of an ideal monatomic gas is raised to at a constant volume. What are the work done by the gas, (b) the energy transferred as heat , (c) the change in the internal energy of the gas, and (d) the change in the average kinetic energy per atom?
