EASY
JEE Main/Advance
IMPORTANT
Earn 100
When is treated with water, it hydrolyses and forms only whereas in acidified aqueous solution forms ion. Explain what is the hybridisation of boron and aluminium in these species?

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
JEE Main/Advance
IMPORTANT
Sum of the pair of electrons on central atom of following species:
HARD
JEE Main/Advance
IMPORTANT
Correct dipole moment order is
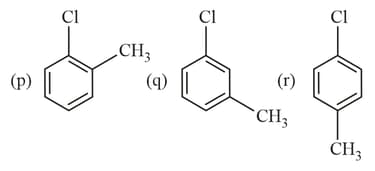
MEDIUM
JEE Main/Advance
IMPORTANT
Arrange the following compounds in decreasing order of their dipole moment
, , ,
EASY
JEE Main/Advance
IMPORTANT
Among the hydrides of group water shows unusual boiling point. Why?
HARD
JEE Main/Advance
IMPORTANT
The correct order of sulphur oxygen bond energy in and is
EASY
JEE Main/Advance
IMPORTANT
Draw the Lewis dot structures of the following compounds:
EASY
JEE Main/Advance
IMPORTANT
Which of the following complexes has a geometry different from others?
MEDIUM
JEE Main/Advance
IMPORTANT
Which of the compound has zero dipole moment?
